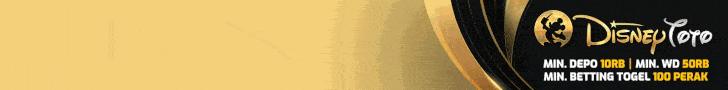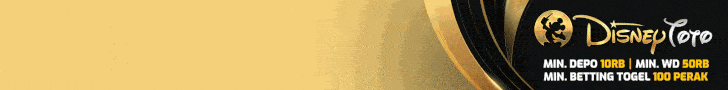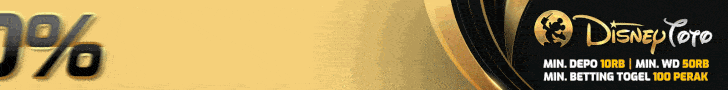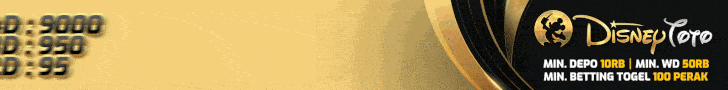Indotogel – Perkiraan Togel Hongkong Hari Ini Jumat Tanggal 08 Maret 2019 memiliki tujuan untuk merumuskan Perkiraan angka jitu Togel Hari Ini kepada anda pencinta togel mania. Pemilihan angka jitu togel anda sebagai pemain togel mania begitu kami harapkan untuk memperoleh hasil yang optimal hingga dapat memperoleh jackpot. Perkiraan yang kami beri dapat digunakan kembali dan diproses kembali supaya memperoleh angka perkiraan yang tepat serta tepat.
Jika pemain togel mania ingin tahu angka perkiraan yang akan keluar pada hari ini di situs Indotogel sebagai referensi tentu bagi anda untuk memperoleh angka yang tepat. Perlu untuk diketahui angka ini ialah prediksi yang kami susun tentunya dengan team ahli kami yang telah memiliki pengalaman dan telah bekerjasama dengan dukun – dukun togel ternama.
Berikut ini kami berikan angka Togel Hongkong Hari Ini Jumat Tanggal 08 Maret 2019 yang telah kami rumus, kami cuma menolong anda memberi angka ramalan saja. Jika ada keselarasan angka dari Indotogel dengan angka dari anda silakan di mainkan angka nya. Anda dapat juga berkunjung ke Fanspage Facebook kami yang akan kami perbaharui sehari-hari nya.
Togel Hongkong Hari Ini Jumat Tanggal 08 Maret 2019
4D : 9 2 2 7
3D : 4 7 0
2D BB : 73, 29, 34, 04, 30, 98
Colok Bebas : 6
Shio : BABI
Buat anda yang saat ini tengah bingung mencari manakah Situs Togel Online yang bagus menjadi patokan anda pada memperkirakan Togel Hongkong Hari Ini Jumat Tanggal 08 Maret 2019 Silakan daftar bersama dengan kami di indotogel melalui banner – banner yang telah kami sediakan. Angka yang kami perkirakan adalah angka yang kami hitung dengan memakai rumusan tepat Togel. Di mana rumusan ini ialah rumusan yang tidak di gunakan pemain togel pada umum nya. Jadi itu bisa kami yakinkan jika perkiraan kami ialah prediksi togel yang sangat tepat diantara yang lainnya. Kami cuma menyiapkan perkiraan buat anda, tergantung anda yang memastikan angka yang ingin anda pasang. Perkiraan yang kami beri ialah perkiraan gratis yang dapat anda lihat dengan setiap harinya.
Demikianlah Prediksi Togel Hongkong Hari Ini Jumat Tanggal 08 Maret 2019 kami buat. Semoga prediksi togel online hari ini bisa memberikan jackpot bagi anda semua. Ikuti kami terus agar anda bisa mendapatkan prediksi togel besok dan seterusnya.
Tag Pada artikel ini :
- Prediksi Togel Hongkong Hari Ini Jumat Tanggal 08 Maret 2019
- Angka Main Togel Hongkong Hari Ini Jumat Tanggal 08 Maret 2019
- Angka Jitu Togel Hongkong Hari Ini Jumat Tanggal 08 Maret 2019
- Indotogel Togel Hongkong Hari Ini Jumat Tanggal 08 Maret 2019
- jbr malam pengeluaran
- Indotogel sgp Hari ini
- data pengeluaran Togel Hongkong Hari Ini Jumat Tanggal 08 Maret 2019
- paito jowo
- data sdy ambarita
- king4d hongkong prize
- Indotogel Cambodia Pools
- Pengeluaran Hongkong Indotogel
data lugano togeltoto - Prediksi togel hari ini
- togel Sidney hari ini
- data sgp
- result sdy
- data hk 4d
- angka keluar hk